
It's used to flavour food and to de-ice roads in the winter. Non-metals can be gases, liquids or solids. The elements which belong to Group VA, VIA, and VIIA are non- metals. They are non-lustrous, brittle and poor conductors of heat and electricity (except graphite). In conclusion, potassium is highly reactive than the sodium. These are electronegative elements with high ionization energies.

Sodium chloride is the most common sodium compound (common salt). Elements that tend to gain electrons to form anions during chemical reactions are called non-metals. Sodium salts, on the other hand, have more applications than the metal itself. Note: In some nuclear reactors, sodium is used as a heat exchanger, and it is also used as a reagent in the chemical industry. Therefore by definition sodium is a metal. Consequently, sodium is a good thermal conductor Heat is moved rapidly from one end to the other by passing energy along in this manner. When you heat a metal, certain ions 'collide' with other ions, causing them to vibrate as well. Most of the metals are hard, have high melting/boiling points, and are malleable as well as. Element Metal/Nonmetal Semi-metal Element Aluminum Metal/Nonmetal Semi-metal Hydrogen Oxygen Tin Silicon Carbon Potassium Sodium. Identify each of the following elements as metal, nonmetal or semi-metal. Test to show metals conduct electricity and non metals do not. The name zirconium is taken from the name of the mineral zircon (the word is related to Persian zargun (zircon zar-gun, gold-like or as gold)), the most important source of zirconium. The ions' vibration is continuous as they keep their place in the lattice. Metallic character is associated with the elements that are metals. This problem has been solved Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Zirconium is a chemical element with the symbol Zr and atomic number 40. Which of the following elements is a nonmetal. Most commonly, it combines with chlorine to form common salt. actinide For the cesium element, give its chemical symbol, atomic number, and group number, and to specify whether each element is a metal, nonmetal, or metalloid. It is highly reactive and is rarely found in nature as a metal. The cations in sodium's metallic structure are similar to one another in a symmetrical geometric pattern. An element has the electron configuration Kr 5s24d105p2. It can conduct electricity well because it has a "sea" of delocalized electrons due to the 1 valence electron, as when a potential difference is applied, mobile charge carriers (electrons) pass from one end of the metal to the other. Since sodium belongs to group 1, it readily loses electrons. A highly reactive and inflammable element, lithium (Li) is the first alkali metal in the periodic table which consists of elements such as sodium (Na). Metals are described as elements that easily lose electrons, are lustrous (reflective), malleable (can be formed into other shapes), and good heat and electricity conductors.
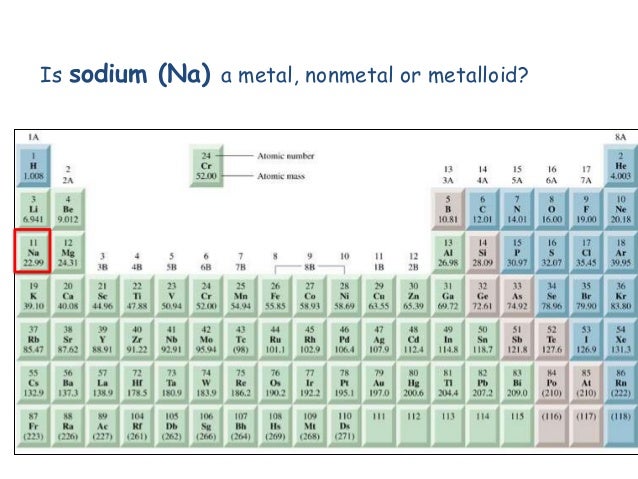
It will assist you in avoiding errors or confusions when answering this question Hint:Before you address this issue, make sure you understand the definitions and properties of metals and non-metals.


 0 kommentar(er)
0 kommentar(er)
